Renu Voice / Renu Gel


Calcium Hydroxylapatite (CaHA)
for long term management of vocal fold insufficiency

Sodium Carboxymethylcellulose (Na-CMC)
for short term management of vocal fold insufficiency
The choice for long- or short-term augmentation
RENÚ Voice is for long-term augmentation:
- A buffered hydrogel with synthetic, proven-biocompatible calcium hydroxylapatite (CaHA) particles 25 to 45 microns in size for consistent and easy injection.1
RENÚ Gel is for short-term augmentation:
- A buffered hydrogel of sodium carboxymethylcellulose2 (Na-CMC) and glycerin.
- Resorbs slowly in vivo, providing a scaffold for local tissue infiltration.3
Higher volume, less waste
Both RENÚ Voice and RENÚ Gel provide a greater volume of material than competitive products: 1.5 cc vs. 1.0 cc.1 The greater volume:
- Helps eliminate waste and inconvenience.
- Reduces the added cost of opening additional syringes.
Convenience and comfort at your fingertips
Both RENÚ Voice and RENÚ Gel are provided in sterile, ready-to-use 1.5 cc syringes with ergonomic finger grips and 2-year shelf life.
Inject RENÚ Voice or RENÚ Gel percutaneously with a needle of your choice or with the RENÚ Double-Bevel, Transoral Needle, supplied separately.3,4
INJECTION OPTIONS:
Operating Room (OR) Trans-Oral Injection
- Visualization via direct laryngoscopy or micro-laryngoscopy
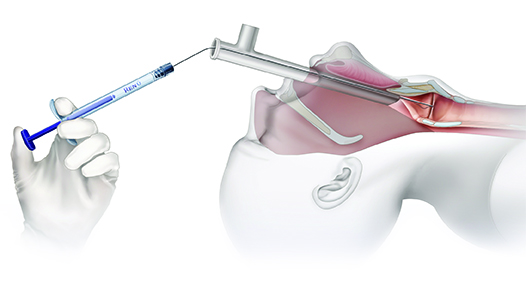
Operating Room Trans-Oral Injections
Office Based Trans-Oral Injection
- Topical anesthesia
- Attach appropriate needle to RENÚ
- Prime needle with RENÚ (dead volume equals ≈0.28cc)
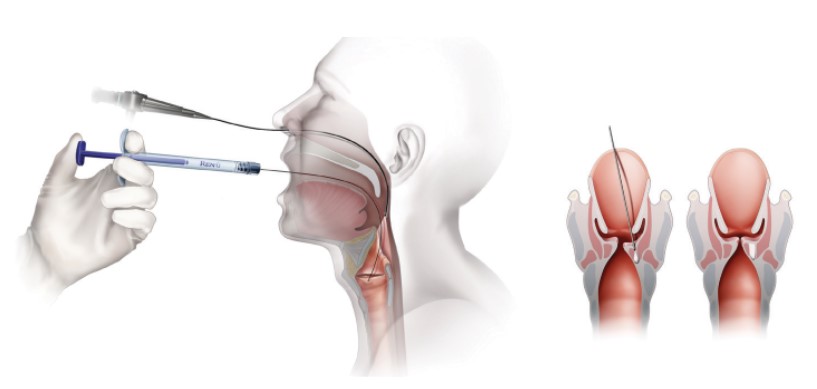
Calcium Hydroxylapatite (CaHA)
Office Based Percutaneous Injections
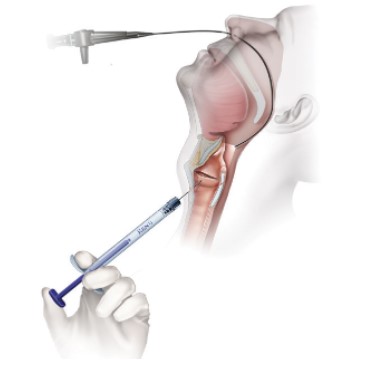
Cricothyroid Cartilage Approach
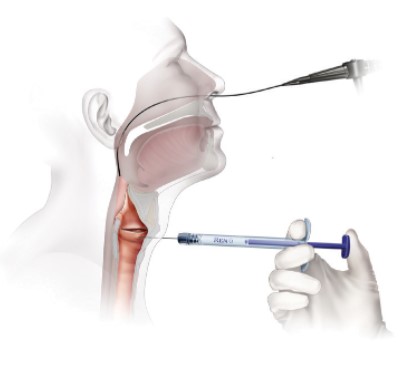
Thyroid Cartilage Approach
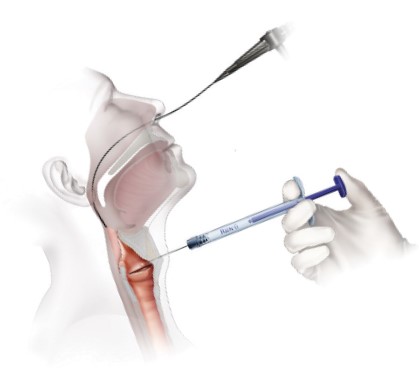
Thyroid Approach
RENU Voice is FDA-cleared and CE-marked for injectable VFI augmentation applications.
RENU Gel is FDA-cleared for injectable VFI augmentation applications.¹
| REF | Description | Rx |
| 08-015-00-V04 | RENÚ Gel Hydrogel Implant, 1.5cc | • |
| 08-015-04-V04 | RENÚ Voice Calcium Hydroxylapatite Implant, 1.5cc | • |
| 08-015-04-VOI | RENÚ Voice Calcium Hydroxylapatite Implant, 1.5cc, CE | • |
| 12-000-00-ND1 | RENÚ Transoral Needle, 24 gauge needle tip, 9.8 inches long, 16 gauge shaft | • |
These products are restricted to sale or use by a licensed professional only.
| Billing Code | Description | Rx |
| 31570 | Direct laryngoscopy with injection (office and OR) | • |
| 31571 | Microlaryngoscopy with injection ( OR only) (microscopic or telescopic) | • |
| 31574 | Laryngoscopy, flexible; with injection(s) for augmentation | • |
| 31575 | Laryngoscopy, flexible; diagnostic | • |
| 31579 | Laryngoscopy, flexible or rigid telescopic, with stroboscopy | • |
| 31599 | Flexible laryngoscopy with injection (trans-oral or percutaneous) | • |
| L8607 | Injectable bulking agent for vocal cord medialization, 0.1 cc | • |
Source: FindACode.com 12.3.19
Support from a trusted global leader
RENÚ Voice and RENÚ Gel are backed with comprehensive support from InHealth Technologies, a global leader.
To order RENÚ Voice and RENÚ Gel, visit https://inhealth.com/products/ent-products/renur.html
References:
1. Data on file, InHealth Technologies.
2. Mallur PS, et al. Safety and efficacy of carboxymethylcellulose in the treatment of glottic insufficiency. The Laryngoscope. 2012;122:322–326. https://doi.org/10.1002/lary.21930.
3. Hughes RGM, Morrison M. Vocal cord medialization by transcutaneous injection of calcium hydroxylapatite. Journal of Voice. 2005;19(4):674-678.
4. Carroll TL, Rosen CA. Long-term results of calcium hydroxylapatite for vocal fold augmentation. The Laryngoscope. 2011;121:313–319.
